The Vaccines would NOT be allowed to bypass FDA approval and have EUA, Emergency Use Authorization if there was any other product available to treat COVID:
https://uscode.house.gov/view.xhtml?req=(title:21%20section:360bbb-3%20edition:prelim)%20OR%20(granuleid:USC-prelim-title21-section360bbb-3)&f=treesort&num=0&edition=prelim
“that there is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating such disease or condition;”
Vaccine companies are given legal immunity for injury or death from vaccines:
https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title42-section300aa-22&num=0&edition=prelim
“No vaccine manufacturer shall be liable in a civil action for damages arising from a vaccine-related injury or death associated with the administration of a vaccine after October 1, 1988, if the injury or death resulted from side effects that were unavoidable even though the vaccine was properly prepared and was accompanied by proper directions and warnings.”
Vaccine companies are given legal immunity if they do not maintain proper product labeling:
https://uscode.house.gov/view.xhtml?req=granuleid:USC-prelim-title42-section300aa-22&num=0&edition=prelim
“No vaccine manufacturer shall be liable in a civil action for damages arising from a vaccine-related injury or death associated with the administration of a vaccine after October 1, 1988, solely due to the manufacturer’s failure to provide direct warnings to the injured party (or the injured party’s legal representative) of the potential dangers resulting from the administration of the vaccine manufactured by the manufacturer.”
The National Vaccine Injury Compensation Program was established which denies people the right to have a jury trial and requires them to petition before a, “special master” who determines if you can be compensated for your vaccine injury:
http://uscfc.uscourts.gov/vaccine-program-readmore
“All vaccine claims are managed and adjudicated by the congressionally created Office of Special Masters, which consists of eight special masters who are appointed to serve for four year terms. The Office of Special Masters is established within the U.S. Court of Federal Claims which appoints and removes the special masters and to which the special masters’ decisions are appealed. A special master has two primary functions: case management, which involves overseeing the collection of information and setting time frames for its submission; and decision making, which involves determining the types of proceedings necessary for presenting the relevant evidence and ultimately weighing the evidence in rendering a final, enforceable decision.”
https://constitution.congress.gov/constitution/amendment-7/
“In Suits at common law, where the value in controversy shall exceed twenty dollars, the right of trial by jury shall be preserved…”
Vaccine companies don’t have to use a true placebo in their trials. They are allowed to use other vaccines as their placebo which can contain harmful ingredients such as mercury, aluminium and other ingredients damaging to humans.
https://www.cdc.gov/vaccines/hcp/conversations/ensuring-safe-vaccines.html
“In Phase 3 studies, hundreds or thousands of volunteers participate. Vaccinated people are compared with people who have received a placebo or another vaccine so researchers can learn more about the test vaccine’s safety and effectiveness and identify common side effects.”
https://penntoday.upenn.edu/news/five-things-know-about-emergency-use-authorization-pfizer-biontech-vaccine
“Another vaccine manufacturer, Sanofi, with a product earlier in development, has indicated that it plans to test its vaccine against one that is already authorized for use rather than a placebo, a more challenging design but one likely necessary to achieve adequate enrollment.’
Science is now saying that random double-blind placebo studies are unethical! Vaccine studies are biased towards approval and require proof they’re unsafe as opposed to proving they’re safe.
https://www.nature.com/articles/d41586-021-00015-0
“Scott Halperin, director of the Canadian Centre for Vaccinology at Dalhousie University in Halifax, who is leading trials of two COVID-19 vaccines in human testing. “Once you have a vaccine that is available,” he notes, “a placebo-controlled trial is no longer ethical or acceptable.””
To get FDA approved, vaccines must prove they’re safe, pure and potent:
https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/frequently-asked-questions-about-therapeutic-biological-products
“What does safety mean? The word safety means the relative freedom from harmful effects, direct or indirect, when a product is prudently administered, taking into consideration the character of the product in relation to the condition of the recipient at the time.”
“Issuance of a biologics license is a determination that the product, the manufacturing process, and the manufacturing facilities meet applicable requirements to ensure the continued safety, purity and potency of the product.”
“What is potency? The word potency is interpreted to mean the specific ability or capacity of the product, as indicated by appropriate laboratory tests, to yield a given result.”
Moderna EUA Fact Sheet:
https://www.fda.gov/media/144637/download
https://www.modernatx.com/covid19vaccine-eua/eua-fact-sheet-providers.pdf
INFORMATION TO PROVIDE TO VACCINE RECIPIENTS/CAREGIVERS
As the vaccination provider, you must communicate to the recipient or their caregiver, information consistent with the “Fact Sheet for Recipients and Caregivers” (and provide a copy or direct the individual to the website www.modernatx.com/covid19vaccine-eua to obtain the Fact Sheet) prior to the individual receiving each dose of the Moderna COVID-19 Vaccine, including:
• FDA has authorized the emergency use of the Moderna COVID-19 Vaccine, which is not an FDA-approved vaccine.
• The recipient or their caregiver has the option to accept or refuse the Moderna COVID-19 Vaccine.
• The significant known and potential risks and benefits of the Moderna COVID-19 Vaccine, and the extent to which such risks and benefits are unknown.
• Information about available alternative vaccines and the risks and benefits of those alternatives.
“The vaccination provider is responsible for mandatory reporting of the following to the Vaccine Adverse Event Reporting System (VAERS):”
“There are no data to assess the concomitant administration of the Moderna COVID-19 Vaccine with other vaccines.”
“Available data on Moderna COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine associated risks in pregnancy.”
“Data are not available to assess the effects of Moderna COVID-19 Vaccine on the breast fed infant or on milk production/excretion.”
“Safety and effectiveness have not been assessed in persons less than 18 years of age.”
Johnson and Johnson EUA Fact Sheet:
https://www.fda.gov/media/146304/download
https://www.janssencovid19vaccine.com/
“FDA has authorized the emergency use of the Janssen COVID-19 Vaccine, which is not an FDA approved vaccine.”
“The significant known and potential risks and benefits of the Janssen COVID-19 Vaccine, and the extent to which such risks and benefits are unknown.”
“There are no data to assess the concomitant administration of the Janssen COVID-19 Vaccine with other vaccines.”
“Available data on Janssen COVID-19 Vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
“Data are not available to assess the effects of Janssen COVID-19 Vaccine on the breastfed infant or on milk production/excretion.”
Pfizer Package Insert:
https://www.fda.gov/media/151707/download
http://labeling.pfizer.com/ShowLabeling.aspx?id=15623
“Postmarketing data demonstrate increased risks of myocarditis and pericarditis, particularly within 7 days following the second dose.”
“COMIRNATY is a vaccine indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older.”
“Postmarketing data demonstrate increased risks of myocarditis and pericarditis, particularly within 7 days following the second dose. The observed risk is higher among males under 40 years of age than among females and older males. The observed risk is highest in males 12 through 17 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. ”
“Available data on COMIRNATY administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
“It is not known whether COMIRNATY is excreted in human milk. Data are not available to assess the effects of COMIRNATY on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for COMIRNATY and any potential adverse effects on the breastfed child from COMIRNATY or from the underlying maternal condition.”
CDC Recommends children 12 and older receive the Pfizer vaccine:
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/adolescents.html
“CDC recommends everyone 12 years and older should get a COVID-19 vaccination to help protect against COVID-19. Widespread vaccination is a critical tool to help stop the pandemic. People who are fully vaccinated can resume activities that they did prior to the pandemic.”
“COVID-19 vaccines are safe and effective.”
“Cases of myocarditis and pericarditis in adolescents and young adults have been reported more often after getting the second dose than after the first dose of one of the two mRNA COVID-19 vaccines, Pfizer-BioNTech or Moderna. These reports are rare and the known and potential benefits of COVID-19 vaccination outweigh the known and potential risks, including the possible risk of myocarditis or pericarditis.”
The American College of Obstetricians and Gynecologists (ACOG) recommendation of COVID vaccines for all pregnant and lactating women:
https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
“The American College of Obstetricians and Gynecologists (ACOG) recommends that all eligible persons greater than age 12 years, including pregnant and lactating individuals, receive a COVID-19 vaccine or vaccine series.”
“COVID-19 vaccines may be administered simultaneously with other vaccines, including within 14 days of receipt of another vaccine. This includes vaccines routinely administered during pregnancy, such as influenza and Tdap.”
“ACOG recommends that lactating individuals be vaccinated against COVID-19. While lactating individuals were not included in most clinical trials, COVID-19 vaccines should not be withheld from lactating individuals who otherwise meet criteria for vaccination. Theoretical concerns regarding the safety of vaccinating lactating individuals do not outweigh the potential benefits of receiving the vaccine, and a growing body of evidence demonstrates that COVID-19 vaccination is safe during lactation (Bertrand 2021, Kachikis 2021). There is no need to avoid initiation or discontinue breastfeeding in patients who receive a COVID-19 vaccine (ABM 2020).”
“Despite ACOG’s persistent advocacy for the inclusion of pregnant individuals in COVID-19 vaccine trials, none of the COVID-19 vaccines approved under EUA have been tested in pregnant individuals. However, studies in pregnant women have begun and post-market surveillance is underway.”
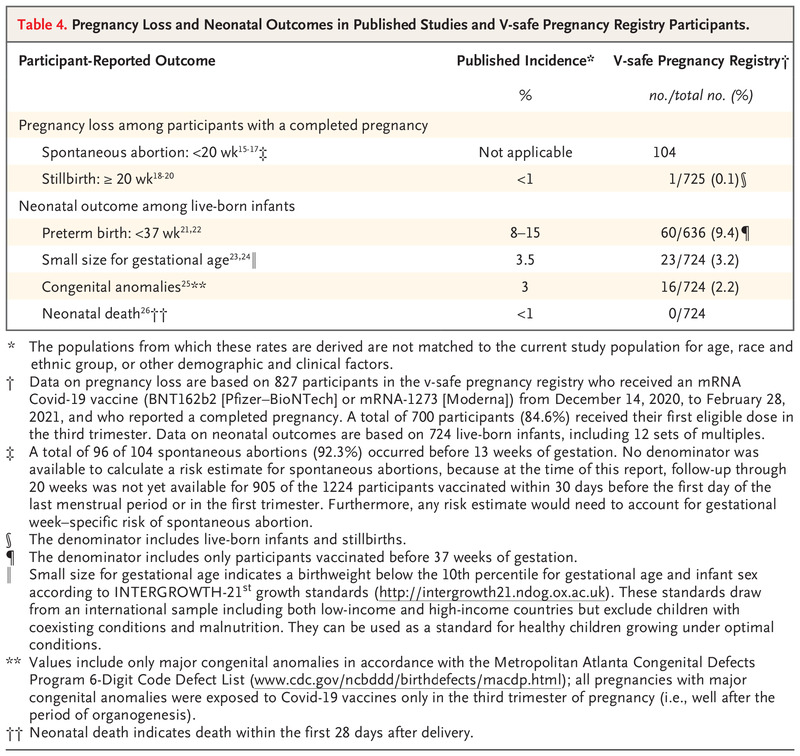
One of the primary studies referenced by ACOG and others to validate safety of Covid vaccination for pregnant mothers was published by the NEJM, New England Journal of Medicine. It justifies that the spontaneous abortion, stillbirth and neonatal outcomes are no different than averages today. However, when looking at their data, the outcomes of spontaneous abortion are seriously flawed and could actually reveal an 81% spontaneous abortion rate:
https://www.nejm.org/doi/10.1056/NEJMoa2104983#
“Among 827 participants who had a completed pregnancy, the pregnancy resulted in a live birth in 712 (86.1%), in a spontaneous abortion in 104 (12.6%), in stillbirth in 1 (0.1%), and in other outcomes (induced abortion and ectopic pregnancy) in 10 (1.2%). A total of 96 of 104 spontaneous abortions (92.3%) occurred before 13 weeks of gestation (Table 4), and 700 of 712 pregnancies that resulted in a live birth (98.3%) were among persons who received their first eligible vaccine dose in the third trimester.”
The total participants who completed pregnancy which includes the baby being born alive, but also includes spontaneous abortion and stillbirth was 827. This study only included mothers who vaccinated at some point in their pregnancy. Spontaneous abortion is defined as a fetal death <20 weeks while a stillbirth is defined as fetal death >=20 weeks. 700 of the 827 received their first vaccine in the third trimester which is 27-40 weeks. This means that those 700 could not possibly be identified in the spontaneous abortion as deaths would have been classified as stillbirth. This means of the 827, only 127 received their vaccine before the third trimester. The study identified 104 spontaneous abortions. 104/127 is 0.8188 or 81.88% spontaneous abortion rate!
ACOG admits when referencing the NEJM study above that they don’t have an, ‘ideal denominator’ for determining the spontaneous abortion rate. This is another way for saying, the denominator we saw in the literature wasn’t ideal.
https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
“It appears that the spontaneous abortion rate following COVID-19 vaccination during pregnancy is consistent with the background rate; however the ideal denominator has not appeared in published literature (Shimabukuro 2021).”
Another report used to justify the need for universal COVID vaccinations for pregnant women was published in the CDC’s Morbidity and Mortality Weekly Report (MMWR) report. This study only reported outcomes of hospitalized pregnant women and did not include healthy pregnancies. The study did NOT address the vaccination rates of those in the study. The overall goal of the report was to say that, “Pregnant women might be at increased risk for severe illness from SARS-CoV-2 infection” and therefore should get the vaccination.
https://www.cdc.gov/mmwr/volumes/69/wr/mm6938e2.htm
“Underlying medical conditions, including obesity and diabetes have been recognized as risk factors for severe COVID-19 disease (7,8). A study of 46 pregnant women with COVID-19 (9) found that nearly all women with severe infection were overweight or obese. This study also identified higher rates of complications in pregnant women with SARS-CoV-2 infection (including the need for ICU admission or mechanical ventilation) and death, which highlight the importance of all pregnant women and their close contacts adhering to COVID-19 prevention measures.”
“The findings in this report are subject to at least five limitations. First, the number of pregnant women with SARS-CoV-2 infection was small, limiting the power to detect significant differences among comparison groups… Fourth, this study did not collect information on prenatal care, which is known to affect pregnancy outcomes… VSD does capture publicly insured persons, and includes one large integrated urban safety-net health system†† serving uninsured patients. Finally, this study did not control for important predisposing factors for adverse birth outcomes, such as pregnancy-related conditions, and more information is needed to understand the effects of SARS-CoV-2 infection on pregnancy outcome.”
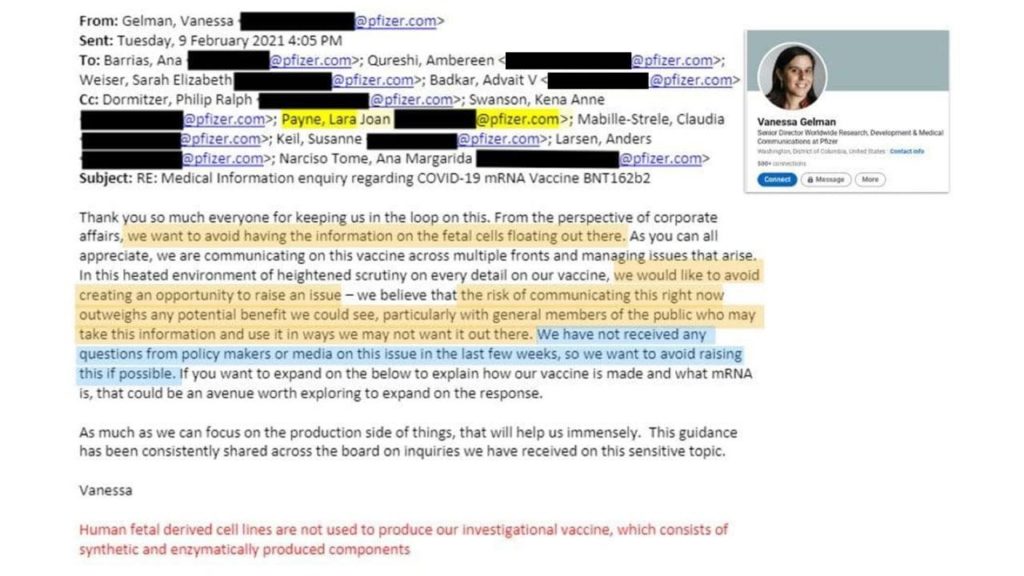
Vaccine Companies have a history of hiding things from the public going all the way up to Vice President and Senior Director:
The importance of this is that there are many people who hold sincerely held religious beliefs to not using products that were tested with or contain cell lines derived from aborted fetal tissue. Admitting this publicly could change public policy and potentially cost the company millions.
“the risk of communicating this right now outweighs any potential benefit we could see, particularly with general members of the public who may take this information and use it in ways we may not want it out there. We have not received any questions from policy makers or media on this issue in the last few weeks, so we want to avoid raising this if possible.”
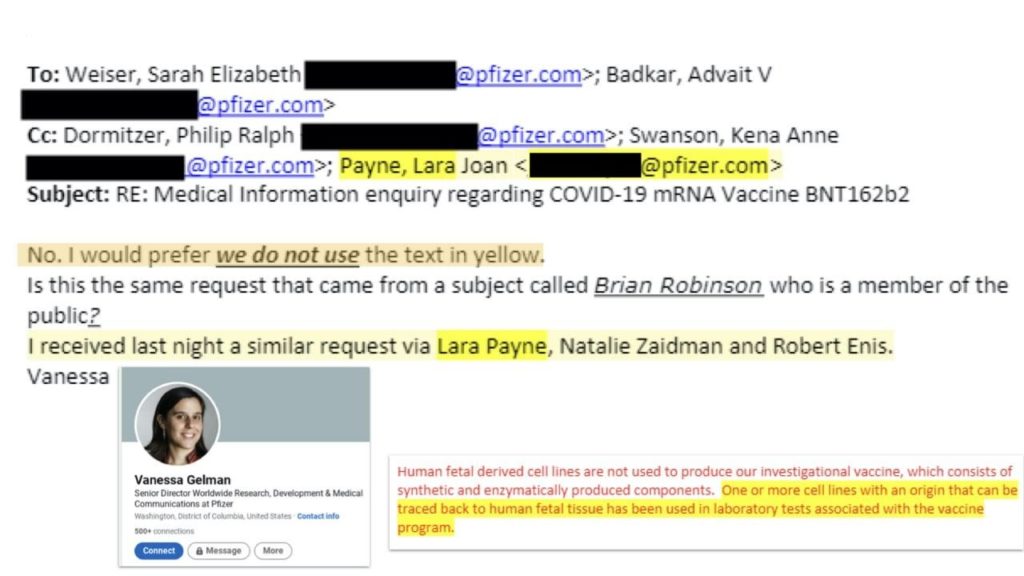
“No, I would prefer we do not use the text in yellow.”
“One or more cell lines with an origin that can be traced back to human fetal tissue has been used in laboratory tests associated with the vaccine program.”
Pfizer to Pay $2.3 Billion for Fraudulent Marketing
https://www.justice.gov/opa/pr/justice-department-announces-largest-health-care-fraud-settlement-its-history
“agreed to plead guilty to a felony violation of the Food, Drug and Cosmetic Act for misbranding Bextra with the intent to defraud or mislead…The company will pay a criminal fine of $1.195 billion, the largest criminal fine ever imposed in the United States for any matter…n addition, Pfizer has agreed to pay $1 billion to resolve allegations under the civil False Claims Act that the company illegally promoted four drugs – Bextra; Geodon, an anti-psychotic drug; Zyvox, an antibiotic; and Lyrica, an anti-epileptic drug – and caused false claims to be submitted to government health care programs for uses that were not medically accepted indications and therefore not covered by those programs. The civil settlement also resolves allegations that Pfizer paid kickbacks to health care providers to induce them to prescribe these, as well as other, drugs. The federal share of the civil settlement is $668,514,830 and the state Medicaid share of the civil settlement is $331,485,170. This is the largest civil fraud settlement in history against a pharmaceutical company.”
Pfizer to Pay $14.5 Million for Illegal Marketing of Drug Detrol
https://www.justice.gov/opa/pr/pfizer-pay-145-million-illegal-marketing-drug-detrol
“The current settlement addresses allegations that Pfizer illegally marketed Detrol, a drug for the treatment of overactive bladder, for use in male patients suffering from benign prostatic hypertrophy and several allied conditions, notably lower urinary tract symptoms and bladder outlet obstruction – all uses for which the Food and Drug Administration (FDA) had not approved the drug as safe and effective.”
The American Red Cross refused to accept Covid vaccinated individuals for their convalescent plasma program. When it came to light, the FDA placed pressure on the Red Cross to alter their criteria and rather than accepting Covid vaccinated people, they shutdown their convalescent plasma program. Somehow past statements were scrubbed and appear to be even from the wayback machine.
his is to ensure that antibodies collected from donors have sufficient antibodies directly related to their immune response to a COVID-19 infection and not just the vaccine, as antibodies from an infection and antibodies from a vaccine are not the same.
https://web.archive.org/web/20210612044557/https://www.redcrossblood.org/content/dam/redcrossblood/docs/covid19_newdonor_vaccine_guide.pdf
“If you receive any type of COVID vaccine, you are not eligible to donate convalescent plasma.”
“One of the Red Cross requirements for plasma from routine blood and platelet donations that test positive for high-levels of antibodies to be used as convalescent plasma is that it must be from a donor that has not received a COVID-19 vaccine. This is to ensure that antibodies collected from donors have sufficient antibodies directly related to their immune response to a COVID-19 infection and not just the vaccine, as antibodies from an infection and antibodies from a vaccine are not the same.”
The FDA slow walked Convalescent Plasma treatment as a viable option to ensure it did not supersede vaccinations. Vaccinations skipped the safety trials that convalescent plasma had to go through so it could come out ahead of other treatments. Vaccinations could not have received emergency use authorization if there were any other drug or treatment available for COVID. Convalescent Plasma has been a time tested solution that has been in use since at least the late 19th century for many viral and bacterial infections.
https://www.history.com/news/blood-plasma-covid-19-measles-spanish-flu
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8226036/
https://www.mayoclinic.org/tests-procedures/convalescent-plasma-therapy/about/pac-20486440
“Data from several clinical trials, studies and a national access program suggest that convalescent plasma with high antibody levels may lessen the severity or shorten the duration of COVID-19 in some people when given early in the disease or in those with weakened immune systems. However, more research is needed to determine if convalescent plasma therapy will be an effective treatment for COVID-19.”
https://www.nih.gov/news-events/news-releases/nih-halts-trial-covid-19-convalescent-plasma-emergency-department-patients-mild-symptoms
“COVID-19 convalescent plasma, also known as “survivor’s plasma,” contains antibodies, or special proteins, generated by the body’s immune system to the novel coronavirus. More than 100,000 people in the United States and many more worldwide have already been treated with it since the pandemic began.”
With the use of convalescent plasma, Covid hospitalization mortality rates went down to 8.6% while Covid hospitalization death rates without it run in the average of 60%.
https://newsnetwork.mayoclinic.org/discussion/mayo-finds-convalescent-plasma-safe-for-diverse-patients-with-covid-19/
“mortality rates declined to 8.6 %”
https://www.cdc.gov/nchs/covid19/nhcs/hospital-mortality-by-week.htm
The FDA in unprecedented moves has recently ignored their advisory boards and approved drugs. Once such example was with an Alzheimer’s drug in November of 2020 where the committee had a votes of 10 no, 1 uncertain and 0 yes. This is basically a unanimous decision, it wasn’t close! The drug in trials appeared to improve cognitive ability by .39 on an 18 point scale which according to the panel members was not statistically significant and did not outweigh the adverse side-effects of the drug. The drug caused brain swelling and bleeding in 32.7% of the treatment group during trials. So a drug that causes a minuscule unnoticeable improvement in cognitive ability and causes life threatening side effects in 32.7% is worth the FDA ignoring its advisory committee and approving it?
https://www.fda.gov/media/145691/download
There have been serious disputes and accusations within the FDA pre-Covid about the FDA senior officials making decisions to approve drugs against their own safety experts and advisory boards:
https://www.documentcloud.org/documents/3911822-206488-Summary-Review-Redacted.html#document/p36/a365992
All government reporting of Covid Cases improperly report everyone as unvaccinated if they have not received 2 doses and are >=2 weeks after their second dose. This has wide implications when you are looking for adverse vaccination events. There are three classifications that should be made, unvaccinated, partially vaccinated and vaccinated. According to the Department of Health & Human Services, Centers for Medicare & Medicaid Services who defines billing and treatment code definitions:
https://www.cms.gov/files/document/qso-20-38-nh.pdf
DEFINITIONS
“Fully vaccinated” refers to a person who is ≥2 weeks following receipt of the second dose in a 2-dose series, or ≥2 weeks following receipt of one dose of a single-dose vaccine.
“Unvaccinated” refers to a person who does not fit the definition of “fully vaccinated,” including people whose vaccination status is not known, for the purposes of this guidance.
The CDC does not properly count for vaccination rates in their reporting which can hide death rates after vaccination by reporting all deaths after first dose up to 2 weeks after the second dose as unvaccinated deaths. This artificially inflates numbers to appear that deaths are because people are unvaccinated and does not account for vaccination deaths.
https://www.cdc.gov/mmwr/volumes/70/wr/mm7034e5.htm
“…unvaccinated <14 days after receipt of the first dose of a 2-dose series or 1 dose of the single-dose vaccine or if no CAIR2 vaccination data were available.”
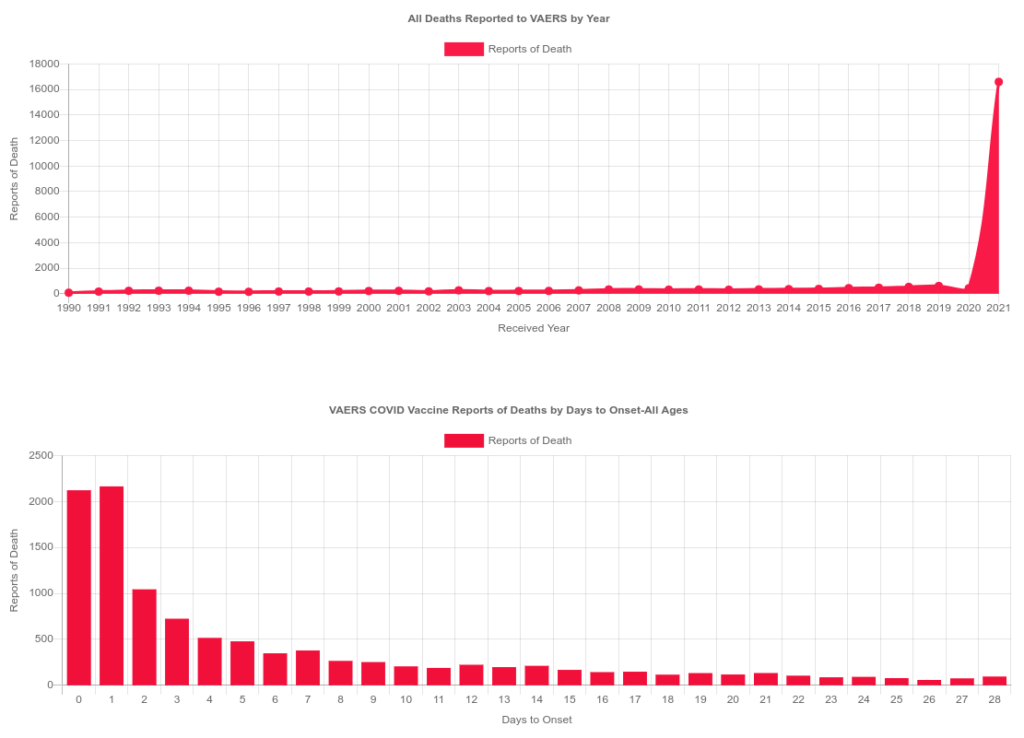
According to VAERS data through October of 2021, there are a significantly high number of deaths several days after COVID vaccinations. Why are there so many spontaneous abortions within the first trimester and why are there so many deaths within the first several days of vaccination that is not characteristic with any other days? Why are the organizations tasked with protecting us from drugs and harm not accounting for obesity, diabetes and other commodities when giving us health guidance? Why was ACOG so willing to throw pregnant and lactating women to be the first ones for medical testing before a product is even approved?
https://openvaers.com/covid-data
People loose control of their reasoning and their perception of reality is changed from true reality when faced with fear. Here is a video showing a key example of how people respond to fear. People will do things that are illogical and could create more harm such as one of the ladies standing away from the boxes and then dropping to them closer to the perceived threat. One of the men actually said multiple times that he felt the bees stinging him. People will deny what they know is right or prudent to do in an emergency to follow the crowd. People will even harm others if they have a higher authority they can defer to for the decision and action of harming others.